In industries where precision, sterility, and contamination control are non-negotiable, cleanrooms serve as the backbone of operations. From pharmaceutical manufacturing and semiconductor fabrication to biotechnology and aerospace, cleanrooms ensure that critical processes are carried out in environments free from unwanted particles. But not all cleanrooms are the same—they’re classified based on how clean the air is inside them. In this article, we’ll dive deep into cleanroom classification systems, industry standards, and the essential role of HVAC systems in maintaining them.
What is a Cleanroom?
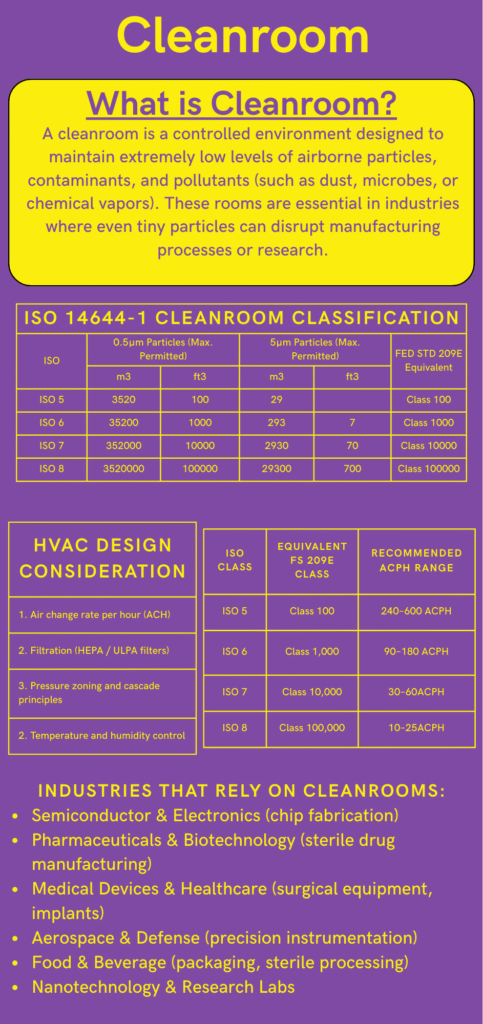
A cleanroom is a controlled environment designed to minimize airborne particles, contaminants, and pollutants to ensure precision in manufacturing, research, and production. Used in industries like pharmaceuticals, semiconductors, biotechnology, and aerospace, cleanrooms maintain strict ISO-classified air quality standards (ISO 14644-1) through HEPA/ULPA filtration, regulated airflow, and contamination control protocols. These specialized spaces prevent defects in microchips, ensure drug sterility, and protect sensitive processes from dust, microbes, and chemical vapors. By controlling temperature, humidity, and pressure, cleanrooms enhance product quality, safety, and regulatory compliance in high-tech and life science applications.

Importance of Cleanroom Classification in Industries
Cleanroom classification is vital across industries to ensure contamination control, product integrity, and regulatory compliance. In pharmaceuticals, strict ISO classifications (e.g., ISO 5 for sterile injectables) prevent microbial contamination and ensure drug safety. The semiconductor industry relies on ultra-clean environments (ISO 1-5) to avoid microscopic defects in microchips, where even a single particle can ruin production. Biotech and medical device sectors require controlled environments (ISO 5-7) to maintain sterile conditions for cell cultures, implants, and diagnostics. Additionally, industries like aerospace, food processing, and nanotechnology depend on cleanroom standards to ensure precision, extend shelf life, and enhance product reliability. Proper classification minimizes risks of contamination, regulatory penalties, and costly recalls, making it indispensable for quality-driven manufacturing.
Role of HVAC in Maintaining Cleanroom Integrity
A cleanroom’s HVAC system is critical for preserving air purity, temperature, and pressure control in contamination-sensitive environments. It ensures HEPA/ULPA-filtered airflow, maintaining ISO-classified particle counts by continuously flushing out contaminants. The system regulates humidity (30-60% RH) and positive/negative pressure to prevent cross-contamination while providing 20-600 air changes per hour (ACH) based on cleanroom class (ISO 5-8). In pharma, semiconductors, and biotech, HVAC stability prevents defects, sterility failures, and compliance risks—making it the backbone of cleanroom integrity.
What is Cleanroom Classification?
Cleanroom classification is categorizing cleanrooms based on the number and size of airborne particles per unit volume of air. The fewer the particles, the higher the classification.
Cleanroom performance is measured using:
- Particle Count: Number of particles of a given size per cubic meter (m³) or cubic foot (ft³) of air
- Air Changes per Hour (ACH): Frequency of air replacement to maintain cleanliness
- Pressure Differentials: Pressure differences between adjacent zones to control contamination
A properly classified cleanroom ensures the required level of cleanliness based on the criticality of the operation being performed.
Cleanroom Classification Standards
- ISO 14644-1 – Global standard (ISO 1 to ISO 9).
- EU GMP Annex 1 – Pharma grades (A/B = ISO 5, C/D = ISO 7-8).
- USP <797> – Sterile compounding (ISO 5 + ISO 7/8).
- FS 209E – Legacy U.S. standard (Class 100 = ISO 5).
- WHO GMP – Combines ISO + microbial limits.
- IEST – Testing & HEPA guidelines.
- SEMI Standards – For semiconductor cleanrooms.
Most industries use ISO 14644-1; pharma prefers GMP grades.
ISO 14644-1: The Global Cleanroom Classification Standard
The most widely adopted cleanroom classification system today is defined in ISO 14644-1, which replaced the outdated Federal Standard 209E.
ISO 14644-1 categorizes cleanrooms from Class 1 to Class 9, but in practical applications, ISO Classes 5, 6, 7, and 8 are most commonly used in industries.
ISO Cleanroom Classification Tables
For Particle Size ≥0.5
| ISO Class | Particle Size (μm) | Max Particles/m³ | Max Particles/ft³ |
| ISO 5 | ≥0.5 | 3,520 | 100 |
| ISO 6 | ≥0.5 | 35,200 | 1000 |
| ISO 7 | ≥0.5 | 352,000 | 10000 |
| ISO 8 | ≥0.5 | 3,520,000 | 100000 |
(Reference: ISO 14644-1:2015)
For Particle Size ≥1.0
| ISO Class | Particle Size (μm) | Max Particles/m³ | Max Particles/ft³ |
| ISO 5 | ≥1.0 | 832 | 123.5 |
| ISO 6 | ≥1.0 | 8,320 | 999 |
| ISO 7 | ≥1.0 | 83,200 | 2,357 |
| ISO 8 | ≥1.0 | 832,000 | 23,500 |
(Reference: ISO 14644-1:2015)
For Particle Size ≥5.0
| ISO Class | Particle Size (μm) | Max Particles/m³ | Max Particles/ft³ |
| ISO 5 | ≥5.0 | 29 | 0.82 |
| ISO 6 | ≥5.0 | 293 | 8.3 |
| ISO 7 | ≥5.0 | 2,930 | 83 |
| ISO 8 | ≥5.0 | 29,300 | 830 |
(Reference: ISO 14644-1:2015)
Application Examples:
- ISO Class 5: Sterile filling of pharmaceuticals, semiconductor lithography
- ISO Class 6: Clean manufacturing for medical devices
- ISO Class 7: Assembly of electronic equipment, injection manufacturing
- ISO Class 8: Packaging, less critical manufacturing steps
Federal Standard 209E: Still in Use
Although Federal Standard 209E was officially withdrawn in 2001, it’s still referenced in many industries and legacy systems.
FS 209E Classification (compared to ISO)
| FS 209E Class | ISO Equivalent |
| Class 100 | ISO 5 |
| Class 1,000 | ISO 6 |
| Class 10,000 | ISO 7 |
| Class 100,000 | ISO 8 |
Many industry professionals continue to use FS terms like “Class 100 Cleanroom” interchangeably with ISO Class 5.
(Reference: Federal Standard 209E, superseded by ISO 14644-1)
Cleanroom Grades in Pharmaceuticals: EU GMP Guidelines
The EU GMP (Good Manufacturing Practices) Guidelines provide a grading system widely used in pharmaceutical cleanroom environments.
Grades A, B, C, and D:
- Grade A: For high-risk operations like aseptic filling. Equivalent to ISO Class 5
- Grade B: Background for Grade A areas, preparation of sterile products
- Grade C: Clean areas for less critical stages
- Grade D: Basic clean areas
Cleanroom classifications in GMP are specified for both “at rest” and “in operation” states, adding another layer of complexity.
(Reference: EudraLex – Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use)
Comparison Table: EU GMP vs USFDA Cleanroom Standards (Particle Counts per m³ and ft³)
| Grade | Particle Size | EU GMP At Rest (m³) | EU GMP At Rest (ft³) | EU GMP In Operation (m³) | EU GMP In Operation (ft³) | USFDA (m³) | USFDA (ft³) |
| A | ≥0.5 µm | 3,520 | 100 | 3,520 | 100 | 3,520 | 100 |
| B | ≥0.5 µm | 3,520 | 100 | 352,000 | 10,000 | 352,000 | 10,000 |
| C | ≥0.5 µm | 352,000 | 10,000 | 3,520,000 | 100,000 | 3,520,000 | 100,000 |
| D | ≥0.5 µm | 3,520,000 | 100,000 | Not Defined | Not Defined | 3,520,000 | 100,000 |
Note: 1 m³ = 35.3147 ft³
(References: EU GMP Annex 1; US FDA Guidance for Industry – Sterile Drug Products Produced by Aseptic Processing)
Types of Cleanrooms by Airflow Design
1. Laminar Flow Cleanrooms
- Unidirectional airflow
- Air flows uniformly in parallel lines
- High air change rate (up to 600+ ACH)
- Used in ISO Class 5 and some Class 6 environments
2. Turbulent Flow Cleanrooms
- Non-unidirectional airflow
- Relies on dilution to maintain cleanliness
- Typically used for ISO Class 6 to 8 cleanrooms
3. Mixed Flow Cleanrooms
- Combination of laminar and turbulent flows
- Balances energy efficiency with cleanliness
(Reference: WHO Technical Report Series 961, Annex 5)
HVAC Design Considerations for Cleanrooms
HVAC systems are critical for achieving and maintaining cleanroom classification. Key factors include:
1. Air Changes Per Hour (ACPH)
Air Changes Per Hour (ACPH):
- ISO 5: 240–600 ACPH
- ISO 6: 90–180 ACPH
- ISO 7: 30–60 ACPH
- ISO 8: 10–25 ACPH
2. HEPA/ULPA Filtration:
- HEPA filters remove ≥99.97% of particles ≥0.3 µm
- ULPA filters remove ≥99.999% of particles ≥0.12 µm
3. Airflow Pattern:
- Laminar flow (unidirectional) for ISO 5–6
- Turbulent or mixed flow for ISO 7–8
4. Pressure Differentials:
- Maintain positive pressure (10–15 Pa) between adjacent rooms
- Helps prevent the ingress of contaminants
5. Temperature and Humidity Control:
- Generally 23°C ± 2°C and 50% ± 5% RH
- Based on product and personnel comfort
6. Return Air Ducts:
- Preferably low-wall returns in ISO 5–7 rooms
7. BMS (Building Management Systems):
- Continuous monitoring of airflow, pressure, temperature, and humidity
8. Cleanroom Layout:
- Minimize cross-contamination by zoning and proper personnel/material flow
Monitoring & Validation
Maintaining cleanroom standards requires continuous monitoring and periodic validation to ensure compliance with ISO 14644 and GMP guidelines.
Key Monitoring Parameters:
- Airborne particle count
- Temperature and relative humidity
- Differential pressure
- Microbial contamination (where applicable)
Common Validation Activities:
- HEPA/ULPA filter integrity test (DOP/PAO test)
- Airflow velocity & volume measurements
- Air Changes Per Hour (ACPH) verification
- Differential pressure measurements
- Temperature and humidity mapping
- Recovery time testing (clean-up time)
- Airflow visualization (smoke test)
- Particle count analysis (ISO 14644-1 compliance)
- Microbiological monitoring (for sterile areas)
- Lighting, noise, and vibration tests (if applicable)
These validation steps help ensure that the cleanroom continues to operate within its designed specifications and maintains its classification during operation.
🔗 Related Resource: HVAC Abbreviations & Terms Every Beginner Must Know (with Cheat Sheet)
References
- ISO 14644-1:2015 – Classification of Air Cleanliness by Particle Concentration
- ISO 14644-2:2015 – Monitoring to Provide Evidence of Cleanroom Performance
- Federal Standard 209E – Airborne Particulate Cleanliness Classes in Cleanrooms and Clean Zones (Archived)
- EU GMP Annex 1 – Manufacture of Sterile Medicinal Products (Revised 2022)
- US FDA Guidance for Industry – Sterile Drug Products Produced by Aseptic Processing
- ASHRAE Handbook – HVAC Applications (Clean Spaces Chapter)
- WHO TRS 961, Annex 5 – WHO Guidelines on HVAC Systems for Pharmaceuticals
Frequently Asked Questions (FAQs)
1. What is the difference between ISO 7 and ISO 8 cleanrooms?
- ISO 7 allows 352,000 particles/m³ (≥0.5 µm), while ISO 8 allows 3,520,000 particles/m³ — a tenfold increase.
- ISO 7 cleanrooms require 30–60 air changes/hour, while ISO 8 typically requires only 10–25 air changes/hour.
- ISO 7 is suitable for tasks like electronic assembly, while ISO 8 is used for packaging or less sensitive processes.
2. How often should cleanroom filters be replaced?
- HEPA filters typically last 1–3 years, depending on usage, differential pressure readings, and air quality.
- Regular integrity testing (DOP or PAO tests) helps determine replacement intervals.
3. Is laminar airflow mandatory for ISO 5 cleanrooms?
- Yes, laminar flow or unidirectional airflow is required in ISO 5 environments (especially for Grade A zones in aseptic pharmaceutical manufacturing) to maintain ultra-low particle levels.
4. What causes pressure imbalance in cleanrooms?
- Leaky doors, improper duct balancing, or exhaust fans running at incorrect speeds.
- Regular HVAC commissioning and balancing is essential to maintain pressure cascades.
5. How is particle count measured in cleanrooms?
- Using laser particle counters that sample air at defined flow rates (usually 1 CFM or 28.3 L/min).
- Sampling is done at critical points, like workstations or air supply zones.
6. What’s the role of airlocks in cleanrooms?
- Airlocks prevent sudden drops in pressure and cross-contamination during personnel or material entry/exit.
- They help maintain pressure gradients between rooms of different classifications.
7. Can an ISO 8 cleanroom be upgraded to ISO 7?
Yes, but it involves:
- Increasing air changes per hour
- Adding more HEPA filters
- Improving sealing, layout zoning, and possibly switching to a better airflow pattern
Conclusion
Cleanrooms are essential for ensuring quality, sterility, and compliance in high-precision industries. Understanding cleanroom classification—whether by ISO 14644-1, EU GMP, or legacy standards like FS 209E—is crucial for selecting the appropriate environment for your process. Equally important is the role of a well-designed HVAC system, which maintains the integrity of cleanroom conditions through controlled airflow, pressure, filtration, temperature, and humidity. By combining robust classification standards with proper HVAC design and routine validation, organizations can safeguard product quality and meet stringent regulatory requirements.

Pingback: SmartHVACGuide.com – Your Trusted Guide to HVAC (Heating, Ventilation & Air Conditioning)